-
Strict Quality Practices
-
New Advanced Instruments
-
Customized Lab Solutions
Welcome to GVP BIO LifeSciences
GVP BIO is providing R&D Products and services to various R&D labs of pharmaceutical companies, research institutes, and organizations. We are specialized in providing Pharmaceutical Impurities (pharmacopeial and non-pharmacopeial) like Process Impurities, API impurities, Degradation Impurities, Potential Impurities and Metabolites, API Intermediates, Building Blocks, Medchem Compounds etc. GVPBIO is a leading manufacturer and supplier of Pharmaceutical API Impurities, API Intermediates & Metabolites.
GVP BIOSCIENCES business model include:
-
API Impurities & & PEPTIDE & Nitrosamine impurities
-
API INTERMEDIATE & FINE CHEMICAL & CRO & CDMO
-
Process Development & Drug Development Support
-
Analytical Services & Deuterated Compounds
Our Services
Impurities, degradants and metabolites of active pharmaceutical and excipients. Isolation, purification and characterisation of high purity compounds and complex molecules. Milligram to multigram scale Analysis to your requirements including NMR, LCMS, GCMS, HPLC, IR and water content. Isolation & Structure Elucidation Of Unknown Impurities Pharmaceutical Reference Standards Contract Research Services Development of new synthetic routes Delivering custom synthesis at off the shelf pricing Process research, development and optimization Cost-effective and high efficiency
Explore MoreProvides high-quality intermediates essential for active pharmaceutical ingredient (API) development. Supplies process-related, degradation, and potential impurities for regulatory submissions. Ensures compliance with global pharmacopeial standards and GMP practices.
Explore MoreOffers diverse chemical scaffolds and reactive intermediates used in drug discovery and chemical development. Enables rapid structure-activity relationship (SAR) studies through ready-to-use compounds. Customized to meet medicinal chemistry and high-throughput screening demands.
Explore MoreSynthesizes drug metabolites for use in preclinical and clinical pharmacokinetic or toxicology studies. Supports both major and minor metabolite production under stringent analytical controls. Facilitates regulatory submissions by providing metabolite reference materials.
Explore MoreDesigns and delivers lead-like molecules to support early-stage medicinal chemistry programs. Supports hit-to-lead and lead optimization with analog synthesis. Backed by SAR-driven synthesis and rapid iteration cycles.
Explore MoreProvides isotopically labeled deuterated compounds for use in ADME studies and drug stability enhancement. Improves pharmacokinetics, metabolic resistance, and intellectual property protection. Supports synthesis of custom deuterium-substituted analogs on demand.
Explore MoreSynthesizes compound libraries targeting specific protein families, pathways, or therapeutic areas. Supports high-throughput screening with structurally diverse yet focused molecules. Accelerates hit identification and early-stage lead discovery.
Explore MoreDevelops efficient synthetic routes for new chemical entities with scalable and cost-effective processes. Optimizes yield, purity, and safety through route selection, risk assessment, and condition refinement. Supports lab-to-pilot scale transition for production readiness.
Explore MoreOur Recent Products

Mycophenolate Mofetil O-Desmethyl Ether
CAT No : GVP-M091060 CAS No : N/A Mol.F. : C22H29NO7 Mol.Wt. : 419.47 Status : Ready Stock Explore More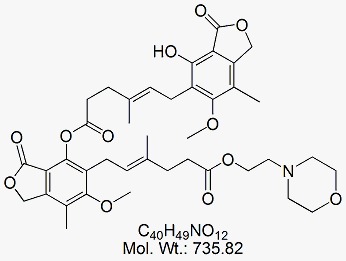
Mycophenolate Dimer
CAT No : GVP-M091059 CAS No : 165684-44-6 Mol.F. : C40H49NO12 Mol.Wt. : 735.82 Status : Ready Stock Explore More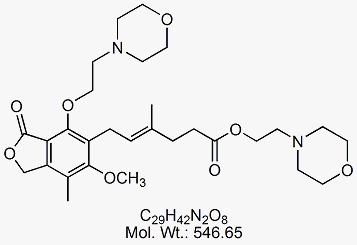
Mycophenolate Di-Mofetil
CAT No : GVP-M091058 CAS No : 868993-14-0 Mol.F. : C29H42N2O8 Mol.Wt. : 546.65 Status : Ready Stock Explore More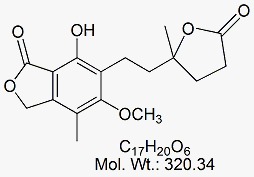
Mycophenolate Mofetil EP Impurity H
CAT No : GVP-M091057 CAS No : 26675-76-3 (rac) ; 79081-87-1 (S-Isomer) Mol.F. : C17H20O6 Mol.Wt. : 320.34 Status : Ready Stock Explore More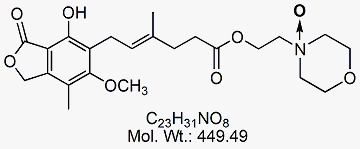
Mycophenolate Mofetil EP Impurity G
CAT No : GVP-M091056 CAS No : 224052-51-1 Mol.F. : C23H31NO8 Mol.Wt. : 449.49 Status : Ready Stock Explore More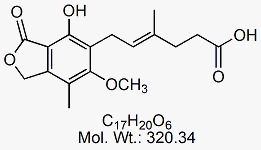
Mycophenolate Mofetil EP Impurity F
CAT No : GVP-M091055 CAS No : 24280-93-1 (acid) ; 37415-62-6 (sodium salt) Mol.F. : C17H20O6 (acid) ; C17H19NaO6 (sodium salt) Mol.Wt. : 320.34 ; 342.32 Status : Ready Stock Explore More
Mycophenolate Mofetil EP Impurity E
CAT No : GVP-M091054 CAS No : 31858-66-9 Mol.F. : C18H22O6 Mol.Wt. : 334.36 Status : Ready Stock Explore More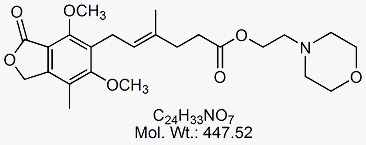
Mycophenolate Mofetil EP Impurity D
CAT No : GVP-M091053 CAS No : 1322681-37-7 Mol.F. : C24H33NO7 Mol.Wt. : 447.52 Status : Ready Stock Explore More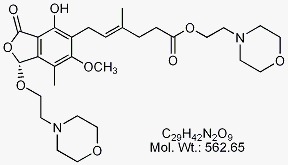
Mycophenolate Mofetil EP Impurity B
CAT No : GVP-M091052 CAS No : 1094322-91-4 Mol.F. : C29H42N2O9 Mol.Wt. : 562.65 Status : Ready Stock Explore MoreOur Clients